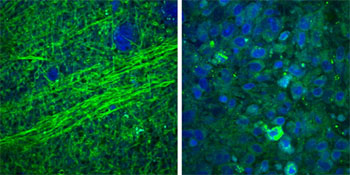
SRS microscopy images show that normal brain tissue contains sparse cells with bundles of nerve axons (left), but brain tumor tissue is full of cells in a disordered pattern (right). Credit: University of Michigan Health System, USA
An international team of physicians and medical school students, led by University of Michigan (USA) neurosurgeon Daniel Orringer, demonstrated in vitro a new quantitative microscope technology based on stimulated Raman scattering (SRS) that could allow surgeons to distinguish normal brain tissue from tumors in real time. The new technique, named SRS microscopy, allowed the researchers to identify lipid-rich brain tissue and protein-rich tumor cells without staining or fluorescent labeling in fresh surgical specimens (Sci. Transl. Med., doi: 10.1126/scitranslmed.aab0195). Removing the entire tumor and sparing healthy brain tissue is important for preventing tumor regrowth and for preserving neurological function.
SRS can reveal differences in tissue cellularity, nerve cell density and protein/lipid ratios by using vibrational properties and macromolecular distribution to generate chemical contrast. For this demonstration, the team collected SRS images at two Raman frequencies (2845 and 2930 cm-1) for each field of view and extracted signals based on Raman intensity ratios for proteins and lipids in brain tissue. The protein signals associated with the clumps of cell bodies found in tumors appeared blue, and the lipid signals associated with the myelinated nerve cell axons in white brain matter appeared green (see image). To ease use during surgery, the researchers developed an automated “classifier” with MATLAB software that can detect these signals automatically so that a surgeon could view the tumor margins while operating.
When compared with traditional tumor staining and imaging, the SRS microscopy method detected tumor infiltration in 22 samples from neurosurgical patients undergoing brain tumor biopsy with 97.5 percent sensitivity and 98.5 percent specificity. Orringer says the new technology “… allows the surgical decision-making process to become data-driven instead of relying on the surgeon’s best guess.” The authors say that if testing continues to go well, they could submit the technique for approval by the U.S. Food and Drug Administration in two years.
