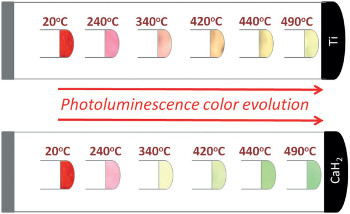
By using different processing temperatures and reducing agents to control the ratio of dopant oxidation states in a single-phase material, CNRS researchers were able to produce phosphorescence at different colors across a red-green range. [Image: G. Kaur Behrh et al., Angew. Chem. Intl. Ed., doi: 10.1002/anie.201504224]
A team from the Centre National de la Recherche Scientifique (CNRS), France, has proposed what it calls “a promising general approach” to chemically tune the photoluminescence colors of single-phase materials by tweaking their oxidation states (Angew. Chem. Intl. Ed., doi: 10.1002/anie.201504224). The researchers believe that the method, called controlled reduction of dopants (CRD), not only could provide a route to better LED color balance, but could find use in controlling a variety of other properties in electronic and optical materials.
LEDs with specific photoluminescence properties, such as creation of white light, commonly are produced by mixing multiple phosphor phases through various chemical means. But the method can sometimes have undesirable chemical side-effects that lead to inappropriate color ratios and unsatisfactory color rendering in many LEDs.
To find an alternative approach, the CNRS team went back to basic redox chemistry. They noted that specific dopants in a given host material will glow at different colors depending on their oxidation state. For example, for phosphorescent systems employing europium (Eu) as a dopant in strontium aluminate (SrAl2O4), the reduced phase, SrAl2O4:Eu2+, shows green luminescence, whereas the oxidized phase, SrAl2O4:Eu3+, shows red luminescence. The researchers reasoned that, by carefully controlling the reduction of the rare-earth dopant through the use of appropriate “oxygen getters” (reducing agents) and temperature, they could generate a phosphor that included a mixture of dopant oxidation states in a single-phase material.
They tested the idea by synthesizing SrAl2O4:Eu3+ using a standard process, putting the material in the company of two oxygen getters, Ti and CaH2, and allowing reduction to take place at controlled temperatures across a gradient from room temperature to 550 ºC. The different temperatures of reduction resulted in different proportions of SrAl2O4:Eu2+ and SrAl2O4:Eu3+ in the material. The result was different colors of photoluminescence as a function of processing temperature, with the specifics dependent on which oxygen getter was used.
The CNRS researchers note that the “soft chemistry” approach of CRD, while used in this case specifically for tuning phosphor color across a red-to-green spectral range, is sufficiently general to be applied to other host materials, such as CsAlSi2O6, to target different emission color ranges (such as red-white-blue) from a single-phase material. And while emission color was the focus here, the scientists believe that the method “could in the future lead to the control of electrical, catalytic, or optical properties of various doped materials.”
