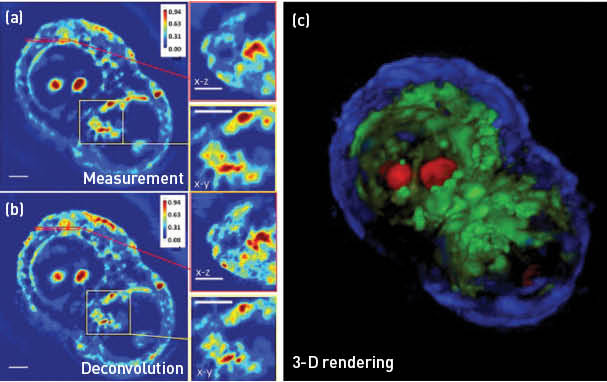
 HT29 cell under mitosis imaged using a 63x/1.4NA objective. (a) Spatial light interference microscopy measurement with a cross- and subsection. (b) White-light diffraction tomography reconstruction with a cross- and subsection, showing increased resolution in 3-D. (c) A false-color 3-D rendering of the same cell, segmented based on the phase values. Scale bars = 5 μm. (Adapted from Kim et al.5)
HT29 cell under mitosis imaged using a 63x/1.4NA objective. (a) Spatial light interference microscopy measurement with a cross- and subsection. (b) White-light diffraction tomography reconstruction with a cross- and subsection, showing increased resolution in 3-D. (c) A false-color 3-D rendering of the same cell, segmented based on the phase values. Scale bars = 5 μm. (Adapted from Kim et al.5)
Living cells are highly transparent under visible illumination, which limits our ability to study them without disruption. Zernike’s phase contrast microcopy has revolutionized cell biology by rendering intrinsic contrast from unlabeled cells.1 However, phase contrast is a qualitative method for visualization. Advances made in image sensors and computing technologies gave birth to a new field called quantitative phase imaging (QPI).2 In essence, QPI combines phase contrast microscopy with holography, which results in a quantitative measurement of the field wavefront. Quantifying optical phase shifts associated with cells and tissues provides a new, valuable dimension to optical microscopy. Over the past decade, QPI has been recognized as an emerging area of research, as it enables previously inaccessible label-free biological applications: cell dynamics, cell growth, blood testing, neuron imaging, cell and tissue refractometry, cell rheology and, more recently, tomographic cell imaging.
Our laboratory made significant advances in QPI in 2014, including quantifying the neural network formation in-vitro, quantifying cancer cell growth and response to drugs and extending QPI to 3-D imaging of unlabeled cells.3–5 In particular, white-light diffraction tomography (WDT) is a novel approach for 3-D imaging of transparent samples. WDT exploits the fact that quantifying amplitude and phase of an imaging field is equivalent to a highly sensitive scattering measurement. Thus, we developed a new inverse scattering solution for broadband illumination and applied it to QPI measurements obtained with white-light interferometry. By scanning the focus through the object and measuring a z-stack, WDT renders a 3-D phase map, which is then processed to image the cell’s structure. When performed with a 63x/1.4NA objective, WDT achieves 350 nm transversal and 890 nm axial resolution. Furthermore, since the imaging system is based on a commercial microscope with a precise incubation system, WDT provides a 4-D imaging capability without damaging the live cell.5
Based on its performance and capability, we anticipate that WDT will become a standard imaging technique in biomedical research, especially in the areas where confocal fluorescence microscopy cannot be used.
Researchers
Gabriel Popescu and Taewoo Kim, University of Illinois at Urbana-Champaign, USA
References
1. F. Zernike. Science 121, 345 (1955).
2. G. Popescu. Quantitative Phase Imaging of Cells and Tissues, McGraw Hill (2011).
3. M. Mir et al. Sci. Rep. 4, 4434 (2014).
4. M. Mir et al. PLoS ONE 9, e89000 (2014).
5. T. Kim et al. Nat. Photon. 8, 256 (2014).
