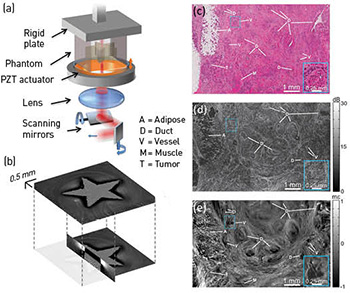
 (a) The OCME sample arm. (b) 3-D micro-elastogram of a phantom containing a stiff, star-shaped inclusion embedded in softer material. (c–e) Histology, en face OCT image and en face micro-elastogram of human breast cancer.
(a) The OCME sample arm. (b) 3-D micro-elastogram of a phantom containing a stiff, star-shaped inclusion embedded in softer material. (c–e) Histology, en face OCT image and en face micro-elastogram of human breast cancer.
Elastography techniques such as atomic force microscopy and ultrasound are widely used in tissue imaging from the nano to macro scales. Yet few of these techniques can probe tissue mechanics on the crucial scale between that of cells and whole organs. Optical elastography—imaging the mechanical properties of tissue using optics—can bridge that gap, providing insights into disease mechanisms and new ways of probing diseased tissue in the clinic.1,2
We have developed optical coherence micro-elastography (OCME), which uses optical coherence tomography (OCT) to map microscale sample deformation in response to mechanical loading into 3-D images of tissue mechanical properties (micro-elastograms).1 Using phase-sensitive OCT, we measure, with nanometer-scale sensitivity, the induced axial displacement in tissue and use this to calculate relative stiffness (local strain) at an axial resolution of 50 to 100 µm. OCME’s transverse resolution matches that of OCT (11 µm in our work).
In 2014, we have brought OCME much closer to clinical translation through innovations spanning applications, novel implementations and theory. We have demonstrated that OCME can identify diseased tissue and provide superior contrast to OCT, and have presented the first micro-elastograms of human breast tissue with clearly identifiable microarchitecture.1 We have further demonstrated that OCME can image the microarchitecture of human lymph nodes1 and can identify muscle damage in a mouse model of muscular dystrophy.3
We have also proposed “optical palpation,” a technique related to tactile imaging methods used for robotic touch, to map the stress response of tissue.4 The technique involves placing a transparent silicone layer between the tissue and the loading mechanism. By tracking the layer’s deformation and knowing its mechanical properties, we map the stress imparted to the tissue, which creates a new imaging modality in its own right. Combined with OCME, optical palpation will soon provide the first maps of absolute stiffness on this scale. Finally, we have developed a multiphysics optical and mechanical model to identify, theoretically, the load that maximizes imaging performance.5
OCME should benefit cancer surgery by enabling clearer delineation and more complete excision of malignant tissue. More generally, it opens a new window on tissue relevant to cell mechanics, tissue engineering and medical imaging.
Researchers
Brendan F. Kennedy, Lixin Chin, Kelsey M. Kennedy, Philip Wijesinghe, Andrea Curatolo, Shaghayegh Es’haghian, Peter R.T. Munro, Robert A. McLaughlin and David D. Sampson, The University of Western Australia, Perth, Australia
References
1. B.F. Kennedy et al. Biomed. Opt. Express 5, 2113 (2014).
2. B.F. Kennedy et al. IEEE J. Sel. Top. Quantum Electron. 20, 7101217 (2014).
3. L. Chin et al. Biomed. Opt. Express 5, 3090 (2014).
4. K.M. Kennedy et al. Opt. Lett. 39, 3014 (2014).
5. L. Chin et al. Biomed. Opt. Express 5, 2913 (2014).
