During the past few decades, there has been a marked decline in tooth decay, or dental caries, in the U.S. population, due to the introduction of fluoride to drinking water, the advent of fluoride dentifrices and rinses, improved dental hygiene, and dietary changes. Yet, in spite of these advances, decay remains the leading cause of tooth loss in the United States, particularly among older adults.
The nature of decay has changed as well. Today, the majority of newly discovered carious lesions are localized to the biting (or occlusal) surfaces of the back teeth and the spaces between teeth—as opposed to the tooth surfaces that face the outside of the mouth, which are more easily detectable. Moreover, modern early carious lesions are often hidden in the complex and convoluted topography of the pits and fissures on the tooth’s surface or concealed by the debris that frequently accumulates on those surfaces.
In the caries process, demineralization occurs as organic acids generated by bacteria diffuse through the porous enamel of the tooth, thereby dissolving the mineral. If the decay process is not arrested, demineralization spreads through the enamel and reaches the underlying dentin, where it rapidly accelerates due to the markedly higher solubility and permeability of dentin. The lesion spreads throughout the underlying dentin to encompass a large area, resulting in the loss of integrity of the tissue and cavitation. At that stage, it is too late for preventive and conservative intervention, and it is necessary to remove a large portion of carious and healthy tissue, often compromising the mechanical integrity of the tooth. If left untreated, the decay will eventually infect the pulp, leading to loss of tooth vitality and possible extraction.
But none of this is inevitable. The caries process is potentially curable, if scientists can identify new diagnostic tools to detect and characterize lesions shortly after they begin. When detected early enough, carious lesions can likely be arrested or reversed noninvasively, using fluoride, anti-bacterial therapy, dietary changes or low-intensity laser irradiation. That’s where optics come in. Optical transillumination, laser-induced fluorescence and optical coherence tomography offer dentists a host of new possibilities for detecting caries early and in the places where it is most commonly found in modern populations.
The need for new solutions
Although the prevalence and nature of caries has changed significantly over the past few decades, the primary diagnostic technologies are basically the same as they were 40 years ago—X-rays and the dental explorer (metal pick). These tools do not work well for the types of lesion most commonly found today. X-rays, for example, are not sensitive enough to detect early lesions, particularly in occlusal surfaces.
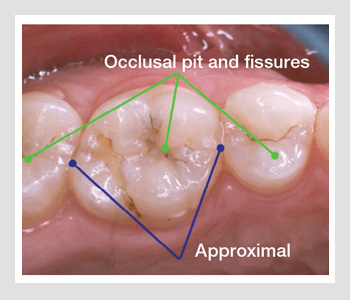 High-risk sites for dental decay. The primary sites for dental decay are in the pits and fissures of the posterior teeth and the approximal contact sites between teeth.
High-risk sites for dental decay. The primary sites for dental decay are in the pits and fissures of the posterior teeth and the approximal contact sites between teeth.
Moreover, by the time the lesions are radiolucent, they have often progressed well into the dentin. Diagnosis by visual and tactile (i.e., with a pick) means are far from ideal as well; visual diagnosis of occlusal caries typically has a very low sensitivity, which can be attributed to the hidden nature of most occlusal lesions. The bulk of the lesion is not accessible and thus is most often not detected unless it is so extensive that it can be resolved radiographically.
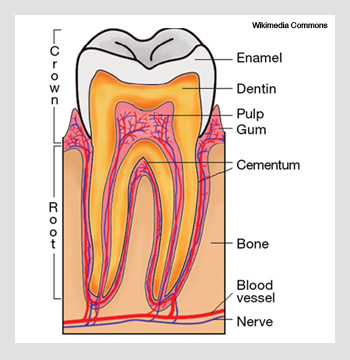
Enamel and dentin—the hard tissues that constitute the tooth—are highly structured in a way that profoundly influences light scattering in those tissues. Dental enamel is an ordered array of rods of inorganic apatite-like crystals surrounded by a protein/lipid/water matrix. The crystals are approximately 30-40 nm in diameter and can be as long as 10 µm. They are clustered together in 4-µm-diameter rods (or prisms), which are roughly perpendicular to the tooth surface.
Dentin is honeycombed with dentinal tubules of 1-3 µm in diameter, each of which is surrounded by a matrix of needle-shaped, hydroxyapatite-like crystals in a protein matrix that is largely composed of collagen. The light scattering distributions of these complex tissues are generally anisotropic and depend on tissue orientation relative to the irradiating light source as well as the polarization of the incident light.
The optical parameters for normal enamel and dentin have been reported in the wavelengths between 200 and 1,550 nm. For enamel, absorption is very weak in the visible range (µa < 1 cm-l, λ = 400-700 nm) and increases in the ultraviolet (µa > 10 cm-l , λ < 240 nm). For dentin in the 400-700 nm wavelength range, the absorption coefficient is essentially wavelength-independent with a value of µa ~ 4 cm-l. Scattering in enamel is strong in the near-UV and decreases ~ λ-3 with increasing wavelength to a value of only 2-3 cm-l at 1,300 nm. In contrast, scattering in dentin is strong throughout the near-UV, visible and near-IR.
During the early stages of demineralization, lesions appear white due to increased backscatter from the demineralized region of early caries lesions. They are called white-spot lesions. Increased porosity of the lesion leads to increased scattering at the lesion surface and higher scattering in the body of the lesion, producing an increase in the magnitude of the backscattered light. At later stages of development, the highly porous lesions acquire increased pigmentation due to the accumulation of chromophores from various bacterial and food byproducts—these are called pigmented lesions.
Optical transillumination
Optical transillumination was used extensively before the discovery of X-rays for detecting dental caries. More recently, the dental community has expressed renewed interest in this method, since high-intensity fiber-optic-based illumination systems have become available for detecting lesions located in-between teeth near the proximal contact points—one of the common locations for caries in today’s population. During fiber-optic transillumination (FOTI), a carious lesion appears dark upon transillumination because of decreased transmission due to increased scattering and absorption by the lesion.
Radiographs are the standard method for diagnosing approximal lesions. Unfortunately, as much as 25 percent of the interproximal areas of radiographs are unresolved due to the overlap with healthy tooth structure on adjoining teeth. Values for sensitivity (0.38 and 0.59) and specificity (0.99 and 0.96) are reported for visual and radiological diagnosis, respectively.
Since dental enamel is virtually transparent at longer wavelengths in the near-IR, this wavelength range is ideally suited for transillumination imaging. Measurements of light scattering in natural lesions at 1,310 nm show that the attenuation increases by two to three orders of magnitude due to demineralization; therefore the optimum contrast between sound and demineralized enamel lies in the near-infrared.
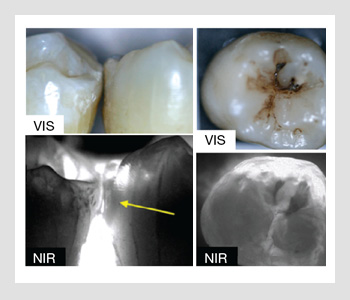 Proximal and occlusal tooth surfaces. (Left) Visible (vis) and near-IR (NIR) light images of the proximal contact point of two extracted teeth placed in contact with a lesion shown by the yellow arrow. (Right) Visible (vis) and near-IR (NIR) light images of the occlusal surface of a tooth with two lesions (dark areas).
Proximal and occlusal tooth surfaces. (Left) Visible (vis) and near-IR (NIR) light images of the proximal contact point of two extracted teeth placed in contact with a lesion shown by the yellow arrow. (Right) Visible (vis) and near-IR (NIR) light images of the occlusal surface of a tooth with two lesions (dark areas).
The figure on the right shows a visible light image of two teeth placed in contact along with a near-IR transillumination image of the contact point taken using a 1,310-nm light source. The enamel is transparent in the near-IR image, and the lesion and dentin appear dark due to the higher light scattering. The lesion is clearly visible with high contrast. The internal incremental growth lines of the enamel are also visible within the transparent enamel.
Researchers have studied other wavelengths, including the region accessible to conventional, less expensive silicon-based CCD cameras—namely 830 nm. However, the maximum contrast lies in the 1,300- to 1,600-nm region. The imaging system operating near 830 nm uses a low-cost silicon CCD optimized for the near-IR. It is capable of significantly higher performance than a visible system, but not of acquiring high contrast images through the full thickness of enamel.
In the near-IR, lesions can also be imaged from the occlusal surface in addition to the traditional approach of imaging through the proximal contact points. This is shown in the figure where near-IR images are taken from the occlusal surface. The images taken from multiple angles allow better characterization of the lesion volume than a simple projection image would. Another advantage of imaging in the near-IR is that many of the chromophores responsible for stains do not absorb in the near-IR and thus do not interfere, allowing for easier discrimination of caries lesions, particularly in the important occlusal surfaces. Near-IR imaging also has great potential for allowing dentists to examine defects in the tooth structure. Moreover, cracks in the enamel can be clearly resolved with this method due to the high transparency of the enamel in the near-IR.
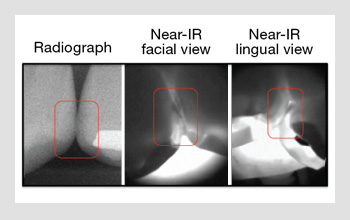 Images of proximal contact points. A bite-wing radiograph and near-IR images of the proximal contact points of two teeth with small lesions acquired in vivo.
Images of proximal contact points. A bite-wing radiograph and near-IR images of the proximal contact points of two teeth with small lesions acquired in vivo.
Recently, we completed the first near-IR clinical study. The figure on the right shows a bite-wing radiograph along with the corresponding near-IR in vivo images taken from the facial and the lingual (tongue side) views. The small lesions are visible with high contrast in the near-IR images.
Approaches that use fluorescence
Teeth naturally fluoresce when they are irradiated with UV and visible light. Alfano and Bjelkhagen et al. demonstrated that laser-induced fluorescence of endogenous fluorophores in human teeth could be used to discriminate between carious and noncarious tissue. Upon illumination with near UV and blue light, yellow-green fluorescence is emitted from sound dental hard tissues. Increased light scattering in lesion areas attenuate the underlying fluorescence and the lesion areas appear dark. This approach yields increased sensitivity for the detection of early lesions and works best on smooth unstained surfaces.
Bacteria produce significant amounts of porphyrins, and dental plaque fluoresces upon excitation with red light. Recently, the Food and Drug Administration approved a new caries detection system called Diagnodent. This low-cost device, which was developed in Kavo, Germany, uses a diode laser and a fiber-optic probe to detect the near-IR fluorescence from porphyrins. It represents a major step toward better caries detection in occlusal surfaces and greatly aids in the detection of hidden occlusal lesions—namely lesions that have progressed into the dentin and that are too small to show up on an X-ray. However, Diagnodent is not designed to detect very early lesions confined to the enamel (the so-called white spot lesions).
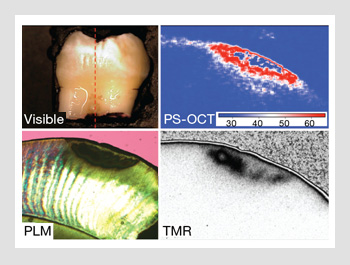 White spot on tooth. An extracted tooth with a white spot lesion on the surface. A cross-polarization image taken along the red dotted line shows the subsurface structure of the lesion. A polarized light micrograph and a high-resolution transverse microradiograph (TMR) are shown of a thin section cut from the tooth in the same position.
White spot on tooth. An extracted tooth with a white spot lesion on the surface. A cross-polarization image taken along the red dotted line shows the subsurface structure of the lesion. A polarized light micrograph and a high-resolution transverse microradiograph (TMR) are shown of a thin section cut from the tooth in the same position.
Optical coherence tomography
Optical coherence tomography (OCT) is a sensitive method for resolving changes in light scattering in early caries lesions. Colston et al. and Feldchtein et al. acquired the first OCT images of soft and hard tissue structures. Polarization sensitivity is particularly useful for imaging caries because early lesions are located at the tooth’s surface. They appear with higher contrast in the cross-polarization images. In addition, the intensity of the strong specular reflection at the tooth surface—which can prevent resolution of the lesion structure—is greatly reduced, and subsurface structure (artifacts) caused by the tooth birefringence can be identified. The figure on the right shows a comparison of the visible reflectance image and the cross-polarization OCT (PS-OCT) image of a tooth with a small white spot lesion. A thin section was cut from the tooth along the red dotted line in the reflected light image; a polarized light micrograph and a high-resolution transverse microradiograph of that section are shown for comparison. The OCT image shows the subsurface structure of the lesion quite well. There is an area of reduced reflectivity near the surface that may indicate that some remineralization of the lesion has occurred. Being able to access the activity of the lesion—i.e., whether it is active and needs intervention or if it has been arrested and can be left alone—is extremely important to the clinician. No currently available diagnostic technology is capable of making such a diagnosis.
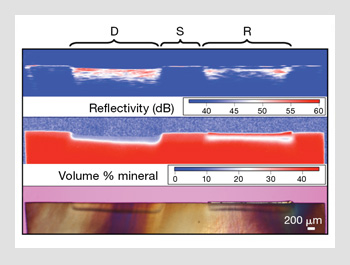 Imaging of the dentin. (Top) Cross-polarization OCT scan of human dentin across a (S) sound area, (D) demineralized area and (R) a similar demineralized area after it was exposed to a remineralizing solution. The remineralized layer is visible as a blue gap (weak reflectivity) near the top of the lesion. The middle and bottom sections of the figure show the corresponding polarized light micrograph and transverse microradiograph imaging from a thin section cut from the sample.
Imaging of the dentin. (Top) Cross-polarization OCT scan of human dentin across a (S) sound area, (D) demineralized area and (R) a similar demineralized area after it was exposed to a remineralizing solution. The remineralized layer is visible as a blue gap (weak reflectivity) near the top of the lesion. The middle and bottom sections of the figure show the corresponding polarized light micrograph and transverse microradiograph imaging from a thin section cut from the sample.
Thus, one of the most exciting potential applications of OCT is the monitoring of remineralization for existing lesions. Initial studies suggest that OCT is ideally suited to that task. The figure on the right shows a polarized light micrograph and a transverse microradiograph of a human dentin sample that was exposed to a demineralization solution (left side) and a demineralization solution followed by a remineralization solution (right side). The lower reflectivity surface layer of remineralized dentin is clearly visible in the PS-OCT image.
Much of operative dentistry consists of replacing existing restorations, and the most common reason for having to replace amalgam and composite fillings is secondary caries. OCT is well suited for detecting secondary caries, since restorative materials have markedly different scattering properties compared to dental hard tissues. Root caries are an increasing problem among our aging population. They are the principal reason for tooth loss in individuals over the age of 44. Lesions on tooth root surfaces can also be measured with OCT.
Other methods
Other potential laser-based methods detecting dental decay include THz imaging, photothermal radiometry and laser-generated ultrasound. Portable THz imaging systems are now available that use pulsed femtosecond lasers and semiconductors. One of the most promising implementations involves THz pulse imaging. This method can be used to measure the remaining enamel thickness to provide a measure of enamel erosion and demineralization.
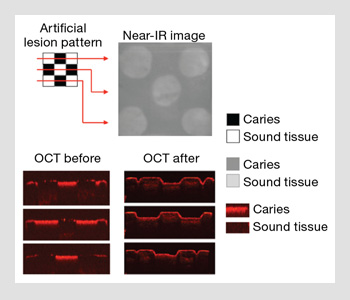 Imaging of the enamel. Near-IR and OCT images of a small 5 x 5 mm block of enamel with patterned areas of demineralization (caries). The areas of demineralization were selectively ablated by a CO2 laser.
Imaging of the enamel. Near-IR and OCT images of a small 5 x 5 mm block of enamel with patterned areas of demineralization (caries). The areas of demineralization were selectively ablated by a CO2 laser.
However, at the present time, these systems are not well suited for in vivo use. Incident temporally modulated laser radiation can be used for frequency-domain photothermal radiometry (FD-PTR). Mandelis and coworkers have applied FD-PTR to detect and analyze dental caries, cracks and other subsurface structures in teeth. Blodgett used laser-produced ultrasonic waves to image internal tooth interfaces. By using a pulsed CO2 laser to produce the ultrasonic waves directly in the tooth, one can overcome the problem of low coupling efficiency to the high-density tooth structure.
Looking forward
In the future, lasers will be used to selectively and precisely remove caries painlessly, while minimizing the loss of healthy tissue. The mineral phase of the surrounding enamel in these high-risk areas may be transformed through laser heating into a more acid-resistant mineral phase to have an enhanced resistance to future decay.
Thus, future patients may not need to endure the current standard of treatment that results in the loss of excessive amounts of healthy tissue structure—which affects appearance and compromises the mechanical integrity of the tooth. Pilot studies on artificial lesions and natural caries lesions suggest that it is feasible to combine imaging methods with laser ablation for highly selective image-guided ablation for removing dental caries. Near-IR transillumination, fluorescence or OCT can be used for image-guided ablation of dental decay. All of this will give both dentists and patients something to smile about.
The author would like to acknowledge Cynthia L. Darling, Michal Staninec, John D.B. Featherstone, Chulsung Lee, Patara Ngaotheppitak, Saman Manesh, Cathy Tao and Ken Fan for their many contributions to this work. The author would also like to thank the NIH/NIDCR for support of his work.
Daniel Fried is with the department of preventive and restorative dental sciences at the University of California, San Francisco, U.S.A.
References and Resources
>> D. Spitzer and J.J. ten Bosch. Calcif. Tiss. Res. 17, 129-37 (1975).
>> R.R. Alfano and S.S. Yao. J. Dent. Res. 60, 120-2 (1981).
>> H. Bjelkhagen and F. Sundstrom. IEEE J. Quantum Electron. 17, 266-8 (1981).
>> J.J. ten Bosch and J.R. Zijp. Dentine and Dentine Research in the Oral Cavity, IRL Press, Oxford, England, (1987).
>> J.D.B. Featherstone and D.G.A. Nelson. Adv. Dent. Res. 1(1), 21-6 (1987).
>> A. Peers et al. Caries Res. 27, 307-11 (1993).
>> D. Fried et al. Appl. Optics 34(7), 1278-85 (1995).
>> C.M. Pine and J.J. ten Bosch. Caries Res 30, 381-8 (1996).
>> A. Schneiderman et al. Caries Res. 31, 103-10 (1997).
>> B.W. Colston et al. Appl. Opt. 37(19), 3582-5 (1998).
>> F.I. Feldchtein et al. Opt. Express 3(3), 239-51 (1998).
>> A. Baumgartner et al. Caries Res. 34, 59-69 (2000).
>> X.Q. Shi et al. Caries Res. 34, 151-8 (2000).
>> R. Hibst et al. Med. Laser Appl. 16, 205-13 (2001).
>> D. Fried et al. J. Biomed. Optics 7(4), 618-27 (2002).
>> R.S. Jones and D. Fried. Lasers in Dentistry VIII, Proc. SPIE 4610, 187-90 (2002).
>> D.W. Blodgett. J. Acoust. Soc. Am. 114(1), 542- 9 (2003).
>> D. Crawley et al. J. Biomed. Opt. 8(2), 303-7 (2003).
>> R.S. Jones et al. Opt. Express 11(18), 2259-65 (2003).
>> G. Jones et al. Lasers in Dentistry X, Proc. SPIE 5313, 17-22 (2004).
>> C.M. Bühler et al. Opt. Express 13(2), 573-82 (2005).
>> P. Ngaotheppitak et al. Lasers Surg. Med. 37(1), 78-88 (2005).
>> C.L. Darling et al. J. Biomed. Optics 11(3), 034023, 1-11 (2006).
>> R.S. Jones and D. Fried. J. Dent. Res. 85(9), 804-8 (2006).
>> R.J. Jeon et al. J. Biomed. Opt. 12(3), 034028 (2007).
>> E. Pickwell et al. Caries Res. 41(1), 49-55 (2007).
>> S.K. Manesh et al. J. Biomed. Opt. 14(044002), 1-6 (2009).
>> Y.-C. Tao and D. Fried. J. Biomed. Opt. 14(054045), 1-6 (2009).
