 Artist’s interpretation of a DNA strand held under tension by two beads trapped in optical tweezers.
Artist’s interpretation of a DNA strand held under tension by two beads trapped in optical tweezers.
It is strange to think that light—that most ephemeral of things—can have any mechanical effect. But it has long been known that the universal palette can, in fact, push and pull on physical objects. The idea was put on sound mathematical footing with the development of the theory of electromagnetism by James Clerk Maxwell, who described what we now call radiation pressure.
Radiation pressure is the most intuitive form of optical force: Light incident on a surface produces a force on that surface. As P.N. Lebedev notes in his experimental verification of this hypothesis, “The value of this beam pressure is rather small.” This is something of an understatement. The maximum pressure exerted by the sun on a reflecting object is on the order of 1 µN/m2. Measuring such a tiny force at the turn of the century, as Lebedev somehow managed to do, was an impressive feat.
The lack of attention that this subject received over the subsequent few decades can perhaps be explained by the fact that it is difficult to disentangle the forces due to the light from thermal forces induced by the light beam. The thermal forces often swamp any effect that one might seek to measure. In addition, the tiny forces that available light sources could generate would also make experiments challenging.
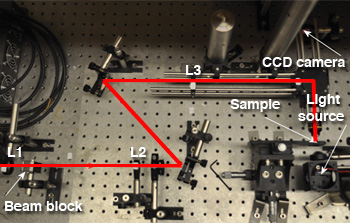 A simple optical tweezers system. The laser input into the system is from the left hand side, indicated by the red line. L1 and L2 form a beam-expanding telescope, as do L3 and L4. Note the system is in a horizontal geometry, in contrast with many optical tweezers that sit in a vertically positioned microscope. The illumination for the sample is provided by a simple white light LED.
A simple optical tweezers system. The laser input into the system is from the left hand side, indicated by the red line. L1 and L2 form a beam-expanding telescope, as do L3 and L4. Note the system is in a horizontal geometry, in contrast with many optical tweezers that sit in a vertically positioned microscope. The illumination for the sample is provided by a simple white light LED.
Ashkin lays the groundwork
Forty years ago, Arthur Ashkin addressed the thermal problem using a relatively new light source called the laser. With it, Ashkin realized that he could use particles that were transparent and therefore non-absorbing at the wavelength of the light source. This de-coupling of optical and thermal forces paved the way for a number of significant breakthroughs. Ashkin was especially interested in developing techniques to manipulate atoms. His work on optical forces culminated in his first observation of the laser cooling of atoms. This application of the technique led to two Nobel Prizes—one given to Steven Chu, Bill Phillips and Claude Cohen-Tannoudji in 1997 for the development of an atomic trap based on laser cooling, and another given to Eric Cornell, Wolfgang Ketterle and Carl Wieman in 2001 for using advanced techniques based on the same principles to create a Bose-Einstein condensate. Laser cooling research is ongoing, with recent breakthroughs in the development of Fermi gases.
Although Ashkin’s research played an important role in the development of these techniques, his best known work is in optical forces on microscopic particles. In this area, he should be rightly seen as having founded a new field. For the bulk of the 1970s, Ashkin and his collaborators embarked on a series of pioneering experiments that showed the capabilities of these optical forces. Of particular importance were demonstrations of trapping using two counter-propagating beams and using a single beam that was obtained by propagating a beam vertically and using gravity to balance the radiation pressure force. Much of Ashkin’s early work was devoted to understanding basic forces and ways to improve stability. However, finding lasting applications or a committed research community proved difficult.
It wasn’t until 1986, when Ashkin demonstrated a single-beam gradient trap, that a more powerful technique was born. The trap differed from the single-beam radiation trap in that it was able to act in the same direction as gravity, forming a true three-dimensional device. In all previous optical trapping work, the gradient force, in which a particle moves along an intensity gradient toward the point of highest intensity, acted only in two dimensions, thereby confining the beam on the beam axis. Trapping in the third dimension—in the direction of the beam propagation—was due to either gravity or the presence of a second counter-propagating beam.
The trick was to focus the beam very tightly to produce a gradient force in the propagation direction that was able to counteract any scattering force. This was achieved by using a high-numerical-aperture microscope objective. Thus, optical tweezers were born. The use of the microscope objective would immediately signal the power of the technique, as it could be coupled to any conventional microscope. In addition to demonstrating simple trapping, the original work also illustrated the general frame of reference for the technique, in which particles within the size regime from 25 nm to 10 µm could be confined.
Optical tweezers
One of the great powers of optical tweezers is the simplicity with which a system can be set up. In fact, basic systems can be developed by motivated high school pupils. This simplicity has aided the take-up of the technique by scientists outside of the optics community. Much more sophisticated systems, including commercial products, are widely used for advanced studies.
Typically an optical tweezers system uses a laser source passed through two telescope systems—the first to expand the beam to slightly overfill the back aperture of the microscope objective and the second to make the beam at the microscope conjugate with the beam on a steering mirror. This helps to aid the optical alignment and allows simple beam steering in the image plane of the microscope objective. A CCD camera can then be used to image the sample plane, and, typically, a sample is loaded on a microscope slide. The choice of laser source is often critical for experiments. For those wishing to look at biological samples, infrared lasers are important due to the reduced photodamage they cause compared to visible light sources. Typical power levels for trapping are not terribly high, with only a few milliwatts of power needed to trap, although most experiments will use tens of milliwatts or more.
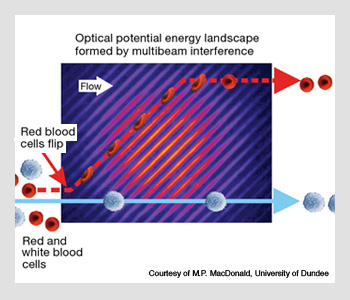 Optical sorting.
Optical sorting.
We have, then, a relatively simple optical system with no strong restrictions on laser source or power levels. But how does it work? The high-school explanation is that light carries momentum that can be transferred to particles as the light passes through them; this transfer results in a force that acts along the intensity gradient in the trapping beam toward the point of highest intensity. A typical laser beam has a Gaussian profile, and so the particle will be drawn toward the beam center. This argument fails for smaller particles, where it is more useful to consider them as dipoles that experience a force within an intensity gradient that results in them being drawn toward the beam center.
This “gradient” force is proportional to the intensity gradient of the beam and also to the polarizability of the particle under consideration. An optical trap to measure forces is a beautiful analog to a simple harmonic oscillator. It has a force relationship in accordance with Hooke’s Law. So, if one can measure displacements of a trapped bead with great accuracy, then forces can be measured with high precision. The trapping forces that can be detected range from a few tens of femtonewtons to the more typical tens of piconewtons to in excess of 100 pN. This range of forces makes optical tweezers an ideal candidate for probing many sensitive biological functions, particularly at the molecular level.
Since the initial single-beam trap was introduced, myriad trapping arrangements have been demonstrated for ever-increasingly complex and sophisticated applications. It quickly became very clear that a single-beam trap was a limiting factor in many cases, and it is very useful in many experiments to have two independent beams. This type of dual-beam trap is simple to set up using beamsplitter arrangements. However, for more complicated trap structures with multiple beams, new techniques had to be introduced.
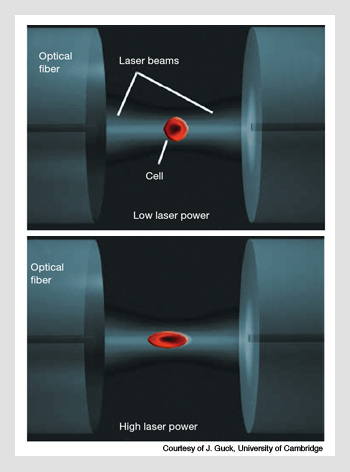 Optical stretching. A cell is trapped between two counter-propagating beams in a dual-beam fiber trap. The laser wavelength used is 1,064 nm. (Top) With low laser power of 200 mW, the cell has no measureable deformation. (Bottom) However, at higher powers of 1.4 W, the cell is appreciably stretched. The amount of stretching is linked to the properties of the cytoskeleton.
Optical stretching. A cell is trapped between two counter-propagating beams in a dual-beam fiber trap. The laser wavelength used is 1,064 nm. (Top) With low laser power of 200 mW, the cell has no measureable deformation. (Bottom) However, at higher powers of 1.4 W, the cell is appreciably stretched. The amount of stretching is linked to the properties of the cytoskeleton.
Multibeam techniques
Currently, there are two multibeam techniques that are widely used—scanning and holographic. In scanning techniques, a beam is scanned very rapidly across the particles of interest. As long as the beam returns to a particle before it diffuses from the trapping site, the particle remains trapped, even if the incident light only illuminates it for a fraction of a second. Perhaps the most famous example of this (if not the most practical) is the microTetris demonstration carried out at the Vrije University.
In holographic techniques, an input Gaussian beam has its phase modulated into that of a target intensity at the focal plane of the microscope objective. In this way, complex beam patterns can be created that are static but dynamically altered. Holographic beam shaping has the advantage that particles can be easily manipulated in three dimensions and that patterns of light that are not simple arrays of spots are relatively easy to produce. As a counterpoint to microTetris, Miles Padgett’s group in Glasgow used holographic optical tweezers to demonstrate particles performing the smallest “Strip-the-Willow” dance in the world.
Scanning and holographic techniques are simply manifestations of scientists’ ability to morph light in complicated ways—in what can be thought of as optical sculpting. They are also, of course, a means to an end. The creation of these tailored landscapes depends primarily on the application in which one is interested. They can be as simple or as complicated as one desires (provided that Maxwell’s equations are not violated!). One of the most interesting applications of shaped light fields in recent years has been that of optical sorting, in which a simple interference pattern is used to separate particles based on some physical property, such as shape, refractive index or size.
The technique, which can sort in the absence of fluorescent markers, works through the simple idea that the optical force imparted to the particle as it flows through the landscape is a function of how the particle interacts with the landscape. As the force is related to physical properties, different particles feel different forces and thus follow varying paths through the light field.
For a macroscopic analog, think of a slanted corrugated roof. If you roll a tennis ball down the roof, it will follow one of the channels formed by the corrugations and move off at the slant angle. A soccer ball, on the other hand, doesn’t “see” the corrugations and thus rolls right over them, falling straight down the roof.
Application-based research
Increasingly, applications are driving optical tweezers research. While there have been a growing number of experiments in areas such as colloidal dynamics, statistical mechanics, hydrodynamics, Brownian motion and nanomanipulation, the biggest market for optical tweezers is in biology. Shortly after Ashkin demonstrated the first optical tweezers, he made use of his new tool to make measurements on cells. This has inspired a huge subfield of work that looks at both cellular and molecular properties.
In some of the most remarkable optical tweezers experiments, researchers have studied the function of single molecules such as molecular motors. The Block group in Stanford has achieved measurements of base pair stepping by RNA polymerase (RNAP), in which an RNAP molecule moves along a DNA molecule; the “steps” taken by the RNAP molecules are approximately 3 angstroms in length. This measurement is phenomenally precise: Each displacement is on the order of the size of a hydrogen atom. The researchers accomplished this task by using a 600-nm polystyrene bead. Their work provides the ultimate example of the application of optical tweezers and shows that real insight can be made at the molecular level.
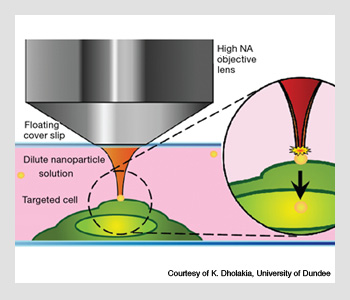 Optical injection of a gold nanoparticle into a cell. The nanoparticle is first trapped by continuous wave optical tweezers. Once in position at the surface of the cell, a pulsed femtosecond beam is applied; this forces the cell through the cell membrane.
Optical injection of a gold nanoparticle into a cell. The nanoparticle is first trapped by continuous wave optical tweezers. Once in position at the surface of the cell, a pulsed femtosecond beam is applied; this forces the cell through the cell membrane.
The field of molecular motor research is now relatively mature. Work on cells is perhaps not as developed. However, as optical physicists start to cross-train in biology, there is significant growth in this area. There is a growing body of work evaluating the properties of blood cells, as these are relatively easy to trap, as well as bacteria. Another growth area is in the spectroscopy of trapped cells, with the easy combination of optical tweezers with any spectroscopic technique that can be put through a microscope. Raman spectroscopy, which often involves long integration times, is of specific interest for those using traps. An additional advantage is that, with trapped cells, it becomes easier to probe sub-cellular and specific sites of interest on the cell membrane.
One of the most interesting developments has been the optical stretcher, in which deformations of cells can be studied in a dual-beam fiber trap. This is particularly useful because the health or properties of a cell can often be linked to the properties of its cytoskeleton. In the case of cancer, it is well known that, in order to proliferate, cells become more “squashy.” The alterations in the cytoskeleton can be detected by the optical stretch, and this makes it possible to use the technique to analyze material from biopsies. Jochen Guck’s group in Cambridge University has recently shown that the optical stretcher can be used to diagnose oral cancer; the researchers simply measured the deformability of the cells. No molecular markers are needed—just a simple mechanical measurement.
Another applied technique that is being invigorated by optical tweezers is that of cell poration—which is typically used for drug delivery. This process primarily involves blasting a cell with a high-intensity pulse of light, typically from a femtosecond laser source. Optical techniques are unlikely to have direct applications for drug delivery in vivo, but they can open the possibility of looking at controlled experiments in vitro and potentially being able to transfect cell lines that are traditionally hard to manipulate. In addition to the transfection of molecular material, these techniques can also be used to controllably introduce nanoparticles into cells—another area of great interest for imaging enhancements and drug delivery.
The technique was developed by Kishan Dholakia’s group at the University of St. Andrews in Scotland. A gold nanoparticle is trapped using optical tweezers and then positioned on the cell surface and injected into the cell using a 100-fs Ti:sapphire laser. This approach opens new avenues in cell microrheology and enhanced Raman spectroscopy, and it may lead to novel strategies for deploying and developing biosensors.
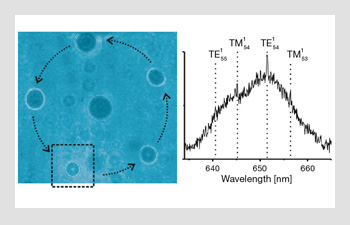 The aerosol carousel. Five aerosol water droplets are trapped in a ring. The droplets can be rotated, using holographic optical tweezers, to move through the “interrogation zone” denoted by the dashed box on the left-hand side. Here a Raman spectra is taken (right-hand image) and can be used to analyze the droplet composition as well as to gain information on its size. Using this technique, one can make comparative measurements between the different droplets
The aerosol carousel. Five aerosol water droplets are trapped in a ring. The droplets can be rotated, using holographic optical tweezers, to move through the “interrogation zone” denoted by the dashed box on the left-hand side. Here a Raman spectra is taken (right-hand image) and can be used to analyze the droplet composition as well as to gain information on its size. Using this technique, one can make comparative measurements between the different droplets
Trapping aerosols
To end, we come full circle. In some of the very first papers published on optical forces in the early 1970s, Ashkin looked at trapping particles in air: aerosols. This optical levitation work did not receive much attention when optical tweezers were developed, and it has remained a niche area that was far removed from mainstream optical manipulation research. It was not until very recently that any airborne particle was actually trapped within a conventional (high-numerical-aperture) optical trap.
Clearly, however, as our need to understand climate change grows, the work on airborne particles is becoming increasingly relevant. Optical tweezers provide a unique way of studying aerosol properties. They are able to localize a range of particle sizes in a simple way that other techniques, such as the electrodynamic balance, cannot match. They are particularly useful in the study of particle dynamics, as they are a non-destructive mechanism—unlike, say, a mass spectroscopy measurement.
In the figure above, we show the combination of some of the techniques we discussed to form an optical carousel for aerosols. Here, a holographic optical tweezers system is used to rotate trapped aerosols through a Raman spectroscopy probe region, so that particles can be analyzed in a comparative fashion, keeping the environment the same but the aerosol size different, for example. Our groups have led the way in analyzing aerosol dynamics, both in understanding the basic physics and technology and in developing techniques to probe atmospherically interesting aerosol properties, such as size, temperature, hygroscopicity, chemical aging and mass accommodation.
These sensitive measurements are made possible by the unique combination of trapping stability, precision control and the optical properties of aerosols themselves. Clearly, the study of aerosols has a long way to go; while we have successfully trapped submicron particles, which are of greater atmospheric interest than the 1-to-10-µm-diameter particles we typically deal with, they are proving a challenge to routinely trap and analyze. The other major challenge will be to examine whether non-spherical transparent liquid particles can be easily trapped. Some evidence indicating that photophoresis works can be used to back up the claim that light can be used to trap such particles, but heating may compromise this approach. Research that evaluates small, opaque particles such as soot would help shed light on ice nucleation and aerosol aging.
The past four decades have seen this optical subfield mushroom into a widely used technique across all the sciences. This explosion of work is unlikely to slow down as more and more physicists become interdisciplinary scientists and move into applied areas in biophysics and chemistry. Technology development continues as well. However, it is the applications—in biology, medicine, atmospheric science, and other areas—that are driving this exciting field. Look for more groundbreaking discoveries in the microworld over the next 40 years.
David McGloin is with the electronic engineering and physics division at the University of Dundee, Dundee, United Kingdom. Jonathan P. Reid is with the School of Chemistry, University of Bristol, Bristol, United Kingdom.
References and Resources
>> MicroTetris
>> Smallest Strip the Willow in the World
>> A. Ashkin. “Acceleration and Trapping of Particles by Radiation Pressure,” Phys. Rev. Lett. 24, 156 (1970).
>> A. Ashkin et al. “Observation of a single-beam gradient force optical trap for dielectric particles,” Opt. Lett. 11, 288 (1986).
>> M.J. Lang and S.M. Block. “Resource letter: LBOT-1: Laser-based optical tweezers,” Am. J. Phys. 71, 201 (2003).
>> D. McGloin. “Optical Tweezers: 20 years on,” Phil. Trans. Roy. Soc. A 364, 3521 (2006).
>> K. Dholakia et al. “Optical Micromanipulation,” Chem. Soc. Rev. 37, 42 (2008).
>> M. Dienerowitz et al. “Optical manipulation of nanoparticles: a review,” J. Nanophot. 2, 021875 (2008).
>> H. Zhang and K.K. Liu. “Optical tweezers for single cells,” J. Roy. Soc. Interface 5, 671 (2008).
>> T. Perkins. “Optical traps for single molecule biophysics: a primer,” Laser & Photon. Rev. 3, 203 (2009).
>> J.B. Wills et al. “Optical Control and Characterisation of Aerosol,” Chem. Phys. Lett. 481, 153 (2009).
