 [Getty Images]
[Getty Images]
All objects exchange energy with their environment. Some of that energy is carried by photons in the form of thermal radiation, or heat—a word sometimes informally used as an antonym for “light,” to highlight thermal radiation’s inherently broadband and incoherent nature, in contrast to, for example, a laser’s coherent, visible light.
Yet a new paradigm is emerging in thermal-radiation control that breaks old stereotypes and the fundamental “laws” we learned at school. This new paradigm arises from the realization that thermal radiation not only can be highly coherent, but can also significantly exceed the limits imposed by Planck’s law. And those insights allow the design of conventional optical elements, such as interference filters, gratings and resonators, that can tailor radiative heat in the same way they currently tailor visible light.
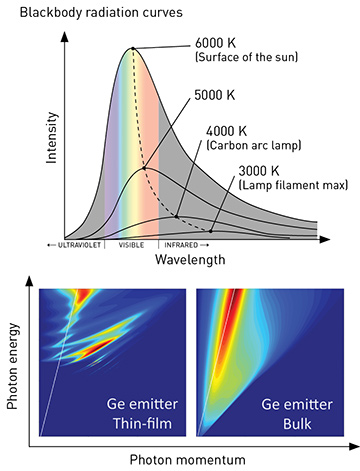 Thermal radiation from bulk materials is spectrally broad, and generally follows Planck’s law (top); spectra of low-dimensional objects such as thin films (bottom left) deviate from those of the same materials in bulk form (bottom right). [J.K. Tong et al., Sci. Reports 5, 10661 (2015)]
Thermal radiation from bulk materials is spectrally broad, and generally follows Planck’s law (top); spectra of low-dimensional objects such as thin films (bottom left) deviate from those of the same materials in bulk form (bottom right). [J.K. Tong et al., Sci. Reports 5, 10661 (2015)]
Blackbodies and real materials
Planck’s law predicts the spectrum and intensity of the thermal radiation emitted by an object at thermal equilibrium, as a function of the object’s temperature. The law also states that the emittance of a nonreflective, completely black object—a so-called blackbody—sets the maximum level of thermal radiation.
The spectral radiance of the blackbody surface emitting photons into a vacuum has a well-known form,
Bλ(λ,T) = (2hc2/λ5)(ehc/(λkBT) – 1)–1,
which depends only on the temperature, T, the wavelength, λ, and universal constants such as the speed of light in vacuum, c, Boltzmann’s constant, kB , and Planck’s constant, h. Under this relationship, the object’s thermal-emission spectrum implies its temperature. That makes Planck’s law an invaluable tool for astronomic observations: very hot objects like the sun emit photons with energies from ultraviolet (UV) to infrared (IR) and beyond, while thermal emission from objects at near-room temperatures is mostly carried by photons with the energies in the mid-to-far-IR range.
Surfaces of real materials, or course, are not completely “black”—nonreflective at all frequencies—and often exhibit spectral radiance reduced by a factor ε(λ,T), known as the spectral emissivity. Spectral emissivity depends on the material composition and typically varies with the wavelength. The emissivity of metals at wavelengths longer than the plasma wavelength, for example, is very low.
Yet bulk material composition is not the only tool for sculpting the thermal-radiation spectrum. Planck himself was well aware that the law bearing his name applies only when “the linear dimension of all parts considered, as well as radii of curvature of all surfaces, … are large compared with the wavelength of the ray considered.” If the thermal emitter’s dimensions are reduced to a length scale comparable to or smaller than the dominant wavelength of the thermal emission set by the emitter temperature, its thermal emission spectrum can deviate significantly from that of the blackbody. And that, in turn, opens up new avenues for harnessing thermal radiation.
A new paradigm is emerging in thermal-radiation control that breaks old stereotypes and the fundamental “laws” we learned at school.
Taming the blackbody
These effects are based on confinement of photons within the emitter volume. The confinement forces the photons to occupy only selected energy states—analogous to the electron confinement in quantum wells, wires and dots that’s used to restrict electron transport in nanostructured materials. Thin films, for example, emit differently from bulk materials of the same composition. Film interfaces thus create thermal wells, which modify the density of optical states available for photons to occupy at a given frequency. Objects of even smaller dimensions, such as nanowires and nanoparticles, can act as thermal wires and thermal dots, with sharp resonant features in their emission spectra due to the excitation of internal optical resonances.
Remarkably, the spectral radiance of thermal wires and dots at the frequencies of their internal optical resonances can exceed the blackbody limit. This is a reciprocal effect of the ability of such objects to “absorb more light than is incident on them,” owing to the strong resonant modification of the local density of optical states in their immediate vicinity. These additional optical states cause bending of propagating light rays around an object, increasing its effective absorption cross-section. They also provide extra channels for the photon emission and cause modification of the factor 2hc2/λ٥ in Planck’s law, which is only valid for the emission of plane waves into vacuum.
Even away from the internal resonances, an emitter’s spectral radiance can be increased by immersing it into a lossless material with a high refractive index n, which serves as a passive heat extractor, increasing the emitted energy flux by a factor of n2. (This is the same physical mechanism—in reverse—that’s used in oil-immersion microscopy to increase a microscope’s resolving power.)
Arranging emitters into ordered arrays—forming gratings, photonic crystals, metasurfaces and metamaterials—allows still further shaping of thermal-emission spectra. This opens up endless opportunities for the optical design of the thermal emission by using the standard toolbox of an optical engineer. The resulting, highly structured emission spectra can even show spectral and spatial coherence.
Finally, the engineering of bulk material properties via optical design can give rise to hyperbolic and epsilon-near-zero, nanostructured photonic metamaterials that show resonant and directional emission spectra. However, far-field thermal emission from nanostructured metasurfaces or metamaterials with surface areas significantly larger than the wavelength of emitted photons can no longer exceed the blackbody limit imposed by Planck’s law.
Minding the (nanoscale) gap
Further challenging Planck’s law in recent decades have been theoretical predictions and experimental demonstrations of radiative heat transfer between objects separated by nanoscale gaps. Those demonstrations have often deviated dramatically from the law’s predictions.
If a thermal emitter is brought close to an absorber, the radiative heat current between them increases beyond the blackbody limit once the emitter–absorber gap becomes much smaller than the wavelength of the dominant radiation—a regime known as near-field radiative heat transfer (NFRHT). If surfaces of the emitter, the absorber, or both support propagating surface polariton modes, the near-field thermal radiation may exceed the blackbody limit by several orders of magnitude.
These surface modes include plasmon polaritons (collective oscillations of free electrons) or phonon polaritons (collective modes resulting from coupling of IR photons with optical phonons). Excitation of surface polariton modes dramatically increases the density of optical states in the gap, which translates into the resonantly enhanced near-field heat flux. Even if the surface polariton modes are not excited, evanescent tails of plane waves undergoing frustrated total internal reflection at the interfaces can boost the energy flux across a vanishing gap by a factor of n2. Understanding and controlling these near-field heat transfer effects offers a key to developing novel devices at the micro- and nanometer scale.
To build devices based on increased NFRHT, however, objects need to be separated by ultranarrow gaps on the order of microns or even nanometers—a limitation that significantly complicates practical device designs. Thus, notwithstanding many theoretical studies, experimental demonstrations of near-field thermal radiation are rather limited. Such demonstrations require maintaining and controlling nanometer-sized gaps while simultaneously measuring heat flows as low as a few picowatts.
In the face of these difficulties, researchers have used a few basic experimental configurations—sphere–plate, tip–plate and plate–plate or parallel-plate—to probe the fundamental laws governing NFRHT. The sphere–plate setup allowed the first successful measurement of NFRHT in sub-100-nm gaps. The tip–plate scheme takes advantage of a scanning probe tip to sample the extreme near field, allowing current theories to be validated in gaps ranging from 10 nm to the sub-nm range. The parallel-plate configuration has proved one of the most difficult geometries to realize experimentally, as it is extremely hard to achieve and maintain perfectly parallel plates at nanometer separations. Very recent work, however, has overcome this long-standing challenge, and achieved a heat current 100 times larger than the blackbody limit between two parallel planar silica surfaces across a 50-nm gap.
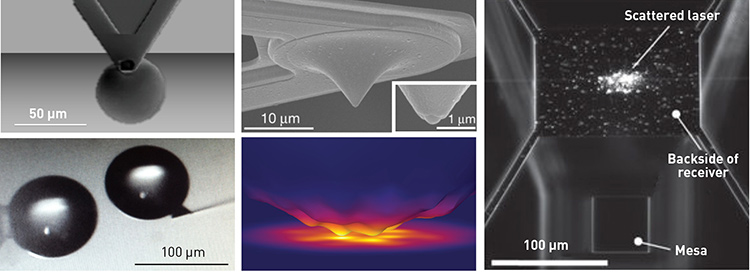 Scanning electron micrographs of NFRHT devices: sphere-to-plate/sphere-to-sphere (left), tip-to-plate (center), and plate-to-plate (right). [S. Shen, et al., Nano Lett. 9, 2909 (2009), K. Kim, et al., Nature 528, 387 (2015), B. Song, et al., Nat. Nanotech. 11, 509 (2016)]
Scanning electron micrographs of NFRHT devices: sphere-to-plate/sphere-to-sphere (left), tip-to-plate (center), and plate-to-plate (right). [S. Shen, et al., Nano Lett. 9, 2909 (2009), K. Kim, et al., Nature 528, 387 (2015), B. Song, et al., Nat. Nanotech. 11, 509 (2016)]
As these preliminary results suggest, NFRHT opens many exciting opportunities to filter, rectify, amplify and modulate radiative heat flow. This work should benefit a broad range of applications including heat-to-electricity energy conversion, development of coherent thermal sources, thermal management and data storage, lithography and thermal microscopy.
Rethinking incandescent lamps
Spectrally filtered thermal emission, delivered via the far field or the near field, is enabling many energy-generation and energy-saving technologies. It has fueled renewed interest in the design of dramatically more energy-efficient incandescent light bulbs. And it holds promise for a more efficient operation of photovoltaic (PV) cells illuminated not by sunlight but by radiation from terrestrial thermal emitters.
Incandescent light bulbs, exemplified by Edison’s lamp, are rapidly being replaced (largely owing to government regulation) by more energy-efficient lighting technologies, such as light-emitting diodes (LEDs). That’s because incandescent emission spectra include a significant portion of IR photons that do not contribute to visible illumination but only to heating the environment. The waste heat significantly reduces the bulb’s overall luminous efficiency, which clocks in at less than 3 percent for a typical incandescent bulb.
Yet those “wasted” IR photons can be made useful again if they can somehow be recycled and re-absorbed by the hot filament. One way to do this is to surround the light bulb with spectrally selective mirrors that allow only the visible part of the thermal-emission spectrum through and that reflect the longer-wavelength, IR part back to the bulb filament. The lamp emits a more intense visible light, yet consumes the same amount of electricity as the standard incandescent lamp.
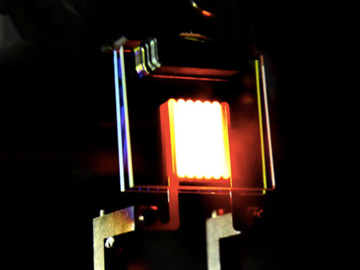 Next-generation incandescent lamps, like this example from MIT, use filters to reflect IR photons back to the filament. [O. Ilic, et al., Nat. Nanotechnol. 11, 320 (2016)]
Next-generation incandescent lamps, like this example from MIT, use filters to reflect IR photons back to the filament. [O. Ilic, et al., Nat. Nanotechnol. 11, 320 (2016)]
The spectrum of the thermal light from an incandescent emitter is more uniform that that of LEDs, and thus more pleasing to the eye. Commercial halogen light bulbs with IR-reflective coatings (HIR lamps) have been on the market for almost two decades already, enabled by technology patented by the General Electric Company. Yet competing with improving LED technology (and meeting stricter government requirements for energy-efficient lighting) will require further innovation. Ongoing research has focused on improving IR-reflecting mirrors and lamp color index, and even on developing filaments that emit less heat. A recent experimental light bulb prototype developed at the Massachusetts Institute of Technology (MIT) boasts 6.6 percent luminous efficiency, well above that of standard incandescent bulbs and at the bottom of the 5-to-20-percent range of commercial LED products. And theory predicts ideal efficiencies of up to 40 percent for these bulbs.
Heat-driven photovoltaics
Photon recycling, and photonic design to sculpt thermal-emission spectra, also lie at the core of thermophotovoltaics (TPV) technology—using terrestrial hot objects as the sources of photons that are converted to electricity by PV cells. These terrestrial thermal emitters may include industrial furnaces used for oil refining or steel and glass making as well as absorbers heated by concentrated sunlight (solar furnaces). It turns out that, notwithstanding their name, typical single p-n junction “solar cells” are not well matched to the broad spectrum of solar radiation; the ideal sunlight-to-electricity conversion efficiency of PV cells is only 41 percent. In a striking contrast, the emission spectra of terrestrial thermal sources can be tailored via optical engineering—and the IR photons can be returned to the emitter to be re-absorbed, keeping it at an elevated temperature.
As a result, theoretical TPV efficiencies are predicted to exceed 50 percent for an ideal p-n junction PV cell with a perfect back-reflector and a blackbody emitter temperature of 1500 K. Spectral tailoring of thermal emission can be highly promising for TPV applications. For example, confined optical modes exhibit cutoff frequencies at longer wavelengths, suppressing emission of photons with energies smaller than the band gap of the PV cell. Other approaches to developing spectrally selective TPV thermal emitters are based on photonic-crystal and optical-metamaterial engineering. The proximity of the PV cell to the terrestrial thermal emitter in a TPV device means that even the sub-bandgap emission can be recycled by using optical filters similar to those used in HIR incandescent lightbulbs.
The possibility of IR photon recycling in TPV systems makes them, at least in theory, an attractive platform for energy conversion that can potentially outperform solar PV cells. But efficient photon recycling is hard to achieve experimentally, because of imperfect back-mirror reflection, parasitic sub-bandgap absorption and sideways radiation losses. Experimental TPV systems include different types of engineered thermal emitters and Si, Ge and InGaAs PV cells. The record experimental efficiency reported to date has exceeded 20 percent for a TPV system with a SiC hot emitter, an InGaAs PV cell and a spectrally selective filter. The highest demonstrated experimental efficiency for a solar-TPV system—in which concentrated sunlight heats a terrestrial absorber, which in turn emits IR radiation toward the PV cell—is around 7 percent. This efficiency, while low, exceeds that of the same cell illuminated directly by sunlight.
Thermally excited emission from PV cells themselves can be used to generate electrical energy. Although the idea is counter-intuitive, “reverse solar cell” systems, or thermoradiative (TR) cells, can generate voltage and electric power via non-equilibrium thermal radiation of IR photons. Heating of a PV cell by a terrestrial heat source, such as waste low-grade heat from a turbine, drives the cell out of equilibrium with the environment, which boosts its emission rate. This process creates a potential difference of opposite polarity from that of a photo-excited solar cell, which can drive electric current through the load.
Although experiments with TR cells have thus far demonstrated only picowatt-level power generation, the technology offers an opportunity to generate clean energy by harvesting radiation from largely untapped terrestrial thermal-emission sources of waste heat—potentially including the Earth itself. Both the energy conversion efficiency and also the power generation density of TPV and TR technologies can potentially be drastically increased via near-field photon extraction.
 [Alessia Kirkland / IR Background: Getty Images]
[Alessia Kirkland / IR Background: Getty Images]
Cooling off
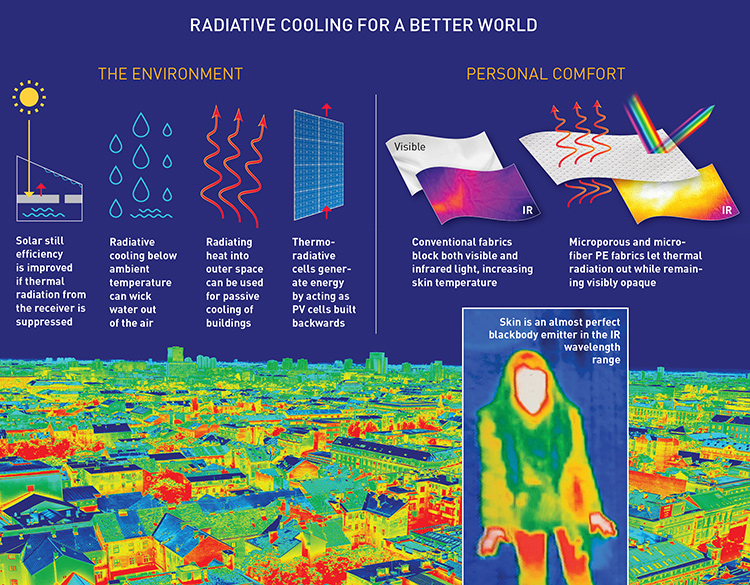
The paragraphs above have largely focused on new ways to harness thermal radiation directly, in applications such as new forms of incandescent lighting or thermophotvoltaic technology. But what about getting rid of some of heat’s negative effects—for example, cooling down on a hot day? Here again, the new focus on thermal-radiation control is suggesting some intriguing, novel engineering to cool buildings, distill water from air, and even create a cooler sports shirt.
Radiative cooling
The increase in waste heat generated as a by-product of energy production, as well as from the rapidly expanding capacity of the computer processors and data networks, has increased the demand for efficient cooling technologies. Recent demonstrations of radiative cooling of terrestrial objects, using the cold of outer space as a heat sink, have paved the way toward achieving this goal. The atmosphere is highly transparent for photons in the 8-to-13-micron frequency range, as no atmospheric gases have significant absorption bands in this range. This “atmospheric-transparency window” allows radiation from terrestrial objects to reach beyond the atmosphere.
To achieve this goal, the terrestrial emitter’s surface needs to be engineered to show high emissivity only in the atmospheric window, with high reflectance outside of that window, to avoid absorption of sunlight and thermal radiation from the atmosphere. Recent experiments have shown that rooftop emitters with vacuum insulation can cool themselves by radiation to as much as 40 °C below ambient temperatures. Even without vacuum insulation, selective-emitter temperatures can be reduced by several degrees below ambient levels. Radiative heat extraction could cool buildings and solar cells even in the daytime with zero energy and water consumption; cooling power in excess of 110 W/m2 have already been demonstrated experimentally.
Solar stills
Temperatures below ambient levels constitute a pre-requisite for dew formation, which is caused by condensation of water vapor from the atmosphere. It occurs naturally during the nighttime under clear skies, as terrestrial surfaces cool down by radiation. Optically engineered surfaces that emit through the atmospheric-transparency window and reject incoming solar radiation can help in addressing the fresh-water shortage in many regions around the globe. Another ancient water-purification technology, solar distillation, can also be improved by thermal-radiation control. In the case of solar stills, reducing thermal emission from the solar receiver used to heat and evaporate contaminated water will increase the still’s overall efficiency. Solar stills with radiation control can trap solar-generated heat so efficiently that they even enable water boiling under direct sunlight.
Self-cooling clothing
One area where terrestrial control of thermal radiation really hits home is in personalized thermal management. Here, new materials and fabrics are emerging that can achieve personal passive temperature control by simply allowing heat to radiate out through clothes rather than being trapped inside.
This emerging technology exploits the frequency-selective filtering of light and heat enabled by scattering on micron-to-sub-micron-scale obstacles. Those obstacles—tuned to minimize reflection and absorption of IR photons, while remaining reflective in the UV, visible and near-IR ranges—can be either fibers in woven and knitted fabrics or pores in porous non-woven materials. Compared with textiles that cool by wicking, these thermally engineered fabrics can provide fully passive means to cool the human body regardless of the person’s level of physical activity. Experiments predict that skin temperatures can be reduced by up to 4 °C if IR-transparent clothes replace conventional cotton and polyester fabrics both for indoor and outdoor use.
Overall, thermal radiation permeates many life processes—from personal thermal comfort to the water evaporation/condensation cycle. Learning how to control it efficiently will prove vital to maintaining and improving the quality of life—and provides a wealth of untapped opportunities for optics and photonics research.
Svetlana V. Boriskina, Hadi Zandavi, Bai Song, Yi Huang and Gang Chen are with the Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, Mass., USA.
References and Resources
-
J.-J. Greffet et al., Nature 416, 61 (2002).
-
X. Liu et al., Phys. Rev. Lett. 107, 45901 (2011).
-
E. Hasman et al., J. Heat Transfer 134, 31023 (2012).
-
J.K. Tong et al., ACS Photon. 2, 769 (2015).
-
D.M. Bierman et al., Nat. Energy 1, 16068 (2016).
-
S.V. Boriskina et al., Nanophoton. 5, 134 (2016).
-
Z. Chen et al., Nat. Commun. 7, 13729 (2016).
-
P.N. Dyachenko et al., Nat. Commun. 7, 11809 (2016).
-
O. Ilic et al., Nat. Nanotechnol. 11, 320 (2016).
-
P.-C. Hsu et al., Science 353, 1019 (2016).
-
W.-C. Hsu et al., Sci. Rep. 6, 34837 (2016).
-
G. Ni et al., Nat. Energy 1, 16126 (2016)
-
Y. Zhai et al., Science 355, 1062 (2017).
